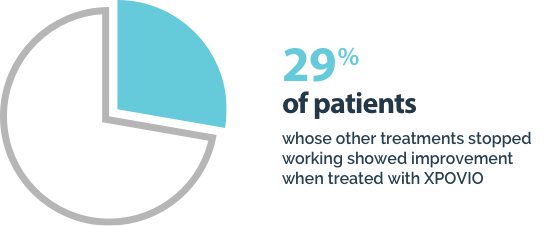
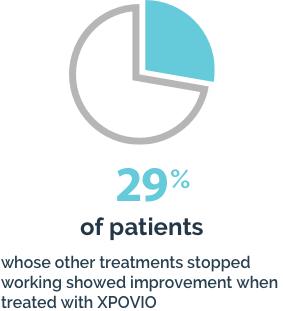
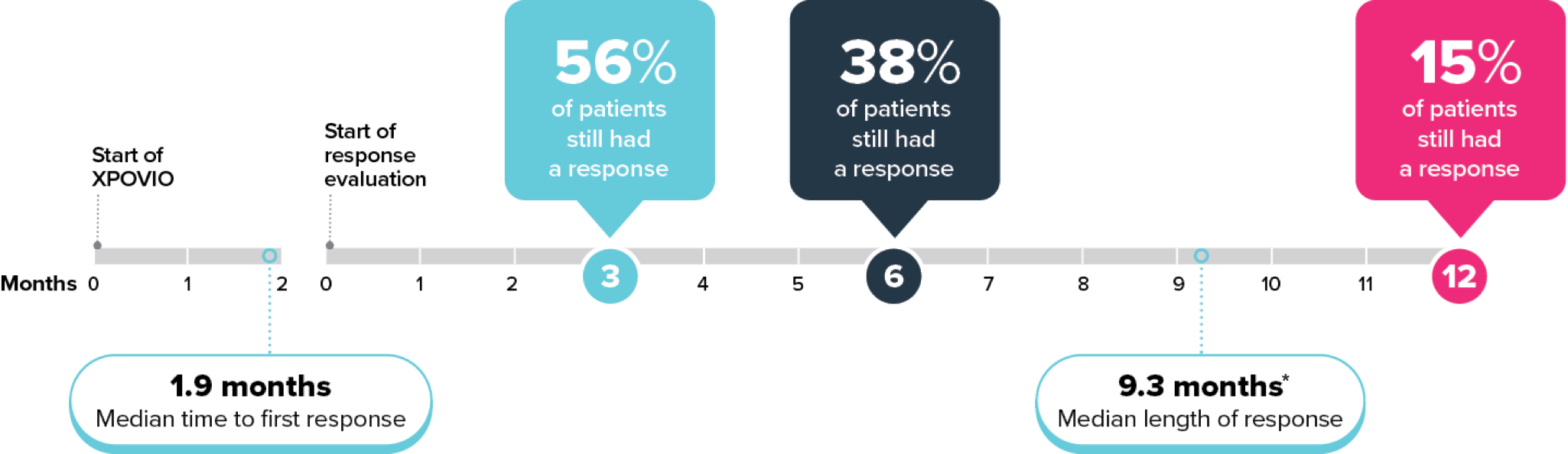
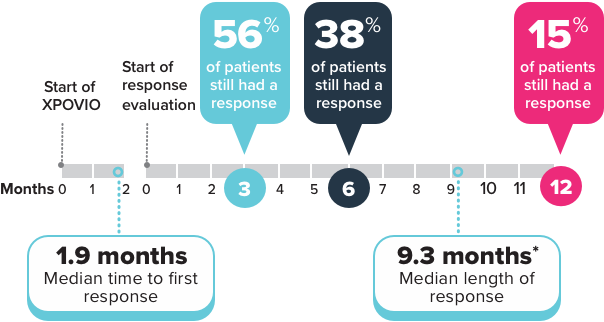
Your healthcare provider will do blood tests before you start taking XPOVIO and often during the first 3 months of treatment and then as needed during treatment.
XPOVIO can cause serious side effects, including:
-
Low platelet counts and low white blood cell counts are common
with XPOVIO and can sometimes be severe or
life-threatening.
-
Low platelet counts can lead to bleeding, which can sometimes
cause death. Your healthcare provider may prescribe platelet
transfusions or other treatments for your low platelet counts.
Tell your healthcare provider right away if you have any bleeding
or easy bruising during treatment with XPOVIO.
-
Low white blood cell counts. You may have an
increased risk of infection during treatment with XPOVIO. Tell your
healthcare provider if you have any signs or symptoms of infection,
including fever, chills, cough, shortness of breath, pain during
urination, or feeling generally unwell. If needed, your healthcare
provider may prescribe antibiotics or certain medicines to help
increase your white blood cell count.
-
Serious infections. XPOVIO can cause infections
that can sometimes cause death, including upper or lower respiratory
tract infections, such as pneumonia, and an infection throughout
your body (sepsis).
Tell your healthcare provider right away if you have any signs or
symptoms of an infection such as cough, chills, or fever during
treatment with XPOVIO.
-
Neurologic side effects. XPOVIO can cause
dizziness, fainting, decreased alertness, and changes in your mental
status, including problems with thinking, seeing or hearing things
that are not really there (hallucinations). These problems can
sometimes be severe and life-threatening. Tell your healthcare
provider right away if you get any of these symptoms and about all
of the medicines you are taking.
Do not drive or operate heavy or dangerous machinery until you
know how XPOVIO affects you. Take precautions to prevent a
fall.
-
Nausea, vomiting and/or diarrhea. Nausea, vomiting
and/or diarrhea can occur when you take XPOVIO and can sometimes be
severe. You may be at risk for becoming dehydrated. Your healthcare
provider may prescribe
anti-nausea or
anti-diarrhea medicines.
-
Loss of appetite and weight loss. Loss of appetite
and weight loss are common with XPOVIO. Tell your healthcare
provider if you have a decrease or loss of appetite and if you are
losing weight.
-
Decreased sodium levels in your blood. Decreased
sodium levels in your blood are common with XPOVIO but can sometimes
be severe or life-threatening. Your healthcare provider may talk
with you about your diet and prescribe IV fluids.
-
New or worsening cataract, cloudiness or loss of transparency of
the lens in the eye.
New or worsening cataract are common with XPOVIO. If a cataract
forms, your vision may decrease, and you may need eye surgery to
remove the cataract and restore your vision.
Tell your healthcare provider right away if you have symptoms of a
cataract such as double vision, blurred vision, or sensitivity to
light or glare.
Common side effects of XPOVIO in adults with multiple myeloma in
combination with bortezomib and dexamethasone include:
- Tiredness
-
Numbness, tingling, or burning in your hands or feet (peripheral
neuropathy)
- Upper respiratory tract infection
- Low phosphate levels in your blood
-
Low red blood cell count (anemia). Symptoms may include tiredness
and shortness of breath.
The most common side effects of XPOVIO in adults with multiple
myeloma in combination with dexamethasone include:
- tiredness
-
low red blood cell count (anemia). Symptoms may include tiredness
and shortness of breath.
- constipation
- shortness of breath
- upper respiratory tract infection
The most common side effects of XPOVIO in adults with DLBCL
include:
- tiredness
- constipation
- fever
-
low red blood cell count (anemia). Symptoms may include tiredness
and shortness of breath.
Ability to have children: XPOVIO may affect the ability of both women and men to have children. Talk to your healthcare provider if you have concerns about fertility.
These are not all of the possible side effects of XPOVIO. Call your doctor for medical advice about side effects. You may report side effects to the FDA at 1‑800‑FDA‑10881‑800‑FDA‑1088.
Before taking XPOVIO, tell your healthcare provider about all of your medical conditions, including if you:
-
have or have had a recent or active infection
-
have or have had bleeding problems
- have or have had liver problems
- have cataracts
-
are pregnant or plan to become pregnant. XPOVIO can harm your unborn baby
-
are taking prescription and over-the-counter medicines, vitamins, and herbal supplements
Females who are able to become pregnant: Your healthcare provider will check to see if you are pregnant before you start taking XPOVIO. You should use effective birth control (contraception) during treatment with XPOVIO and for 1 week after your last dose. Tell your healthcare provider right away if you become pregnant or think you might be pregnant during treatment with XPOVIO. Do not breastfeed during treatment with XPOVIO and for 1 week after your last dose of XPOVIO. It is not known if XPOVIO passes into your breast milk.
Males with female partners who are able to become pregnant should use effective birth control (contraception) during treatment with XPOVIO and for 1 week after your last dose.
Please see the Medication Guide and the full Prescribing Information for XPOVIO.
To report SUSPECTED ADVERSE REACTIONS, contact Karyopharm Therapeutics Inc. at 1‑888‑209‑93261‑888‑209‑9326 or FDA at 1‑800‑FDA‑10881‑800‑FDA‑1088 or www.fda.gov/medwatch.